Resources and Tools
Biobank Samples & Data Directory
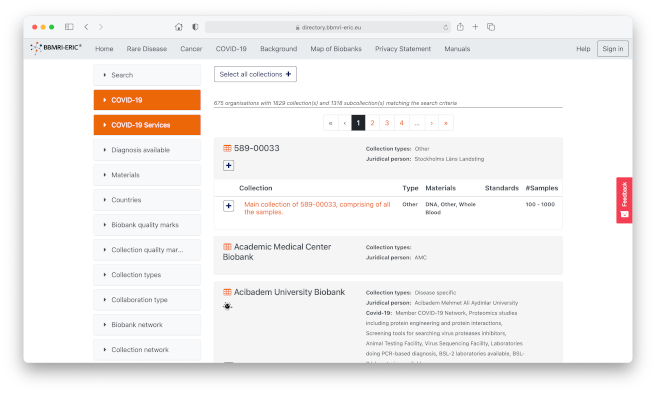 Are you looking for samples and clinical data for your biomedical research? The BBMRI-ERIC Directory makes it easy, with a catalogue of samples and data from nearly 700 biobanks across Europe – and, since 2020, those with samples and data related to COVID-19 from around the world.
Are you looking for samples and clinical data for your biomedical research? The BBMRI-ERIC Directory makes it easy, with a catalogue of samples and data from nearly 700 biobanks across Europe – and, since 2020, those with samples and data related to COVID-19 from around the world.
Once you’ve found your samples, send inquiries to multiple biobanks using our Negotiator tool. The Negotiator provides an easy overview of the status of your requests and allows tracking of follow-up steps and cost negotiation.
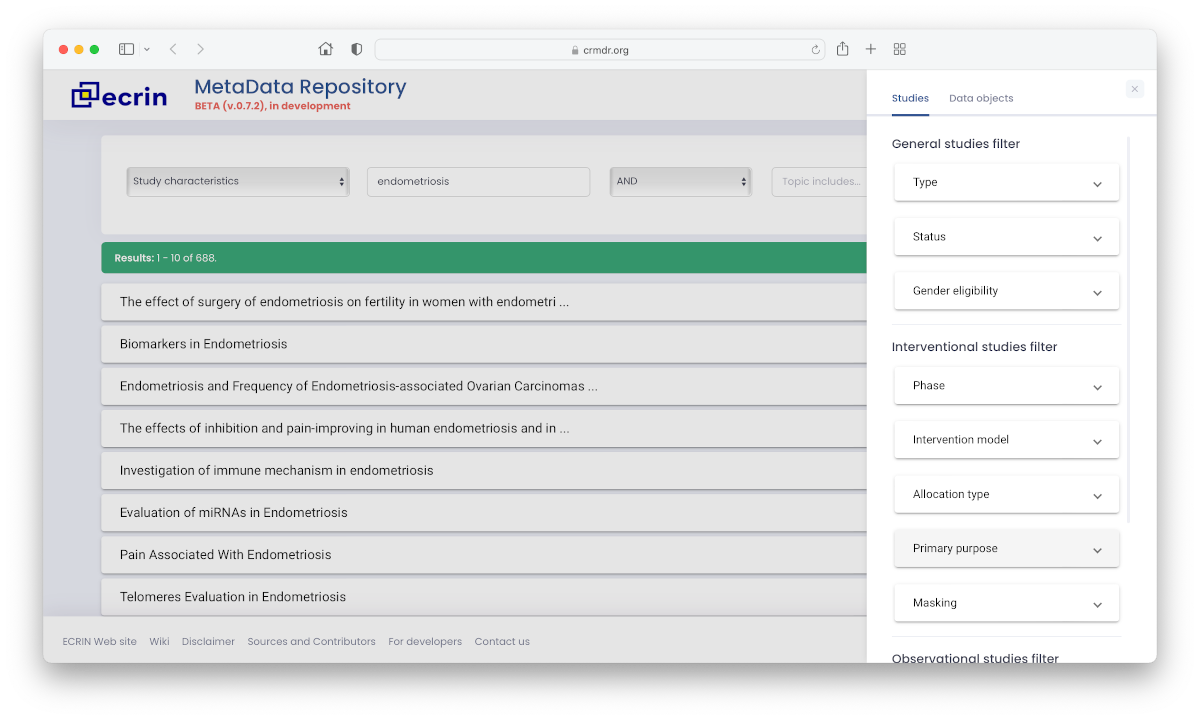
Clinical Research Metadata Repository
The online tool to help scientific researchers find documents and data linked to a clinical research study, and to obtain information on the accessibility of those results. The Clinical Research Metadata Repository is freely available for all scientific researchers, and is updated regularly through collection of data from the most important sources of information worldwide.
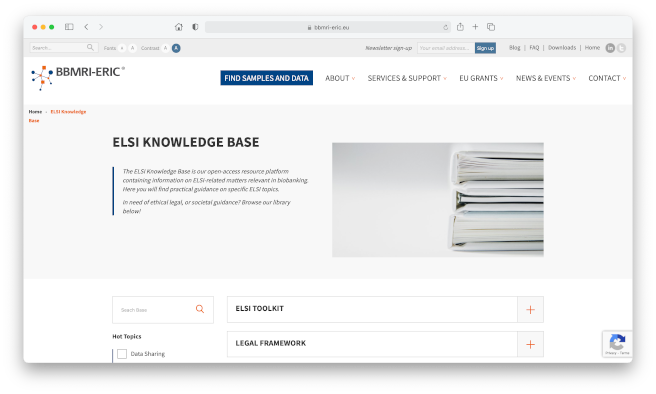
ELSI Knowledge Base
The ELSI Knowledge Base is the open-access resource platform containing information on ELSI-related matters relevant in biobanking and life sciences. Here you will find practical guidance on specific ELSI topics. So if you are in need of ethical legal, or societal guidance, please browse the library at the link below!
Regulatory tools

Through a combination of in-house and external partnerships with a range of regulatory experts and groups, EATRIS can provide regulatory support for most types of product. The EATRIS Regulatory Database is free of charge, and contains information about the regulatory requirements, guidelines and legislations from 27 EU countries (and Norway, Switzerland, Turkey and Israel).
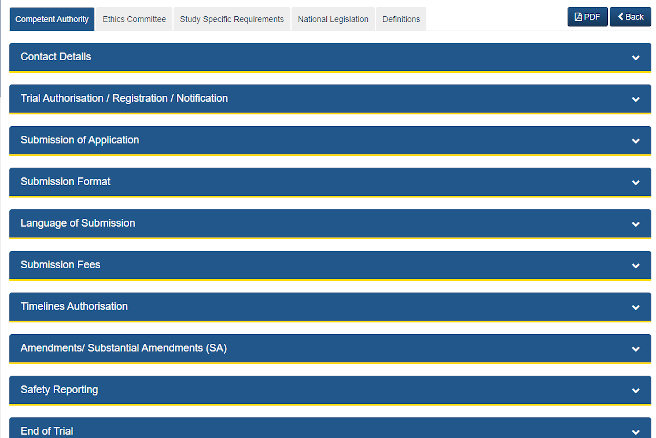
ECRIN CAMPUS is a central resource for information about clinical trial regulatory and ethical requirements covering 22 European countries and multiple study types. Use CAMPUS to locate country-specific competent authorities and ethics committees, consult the summary of requirements for each country, compare country information, and browse related documents.
Go to ECRIN Campus